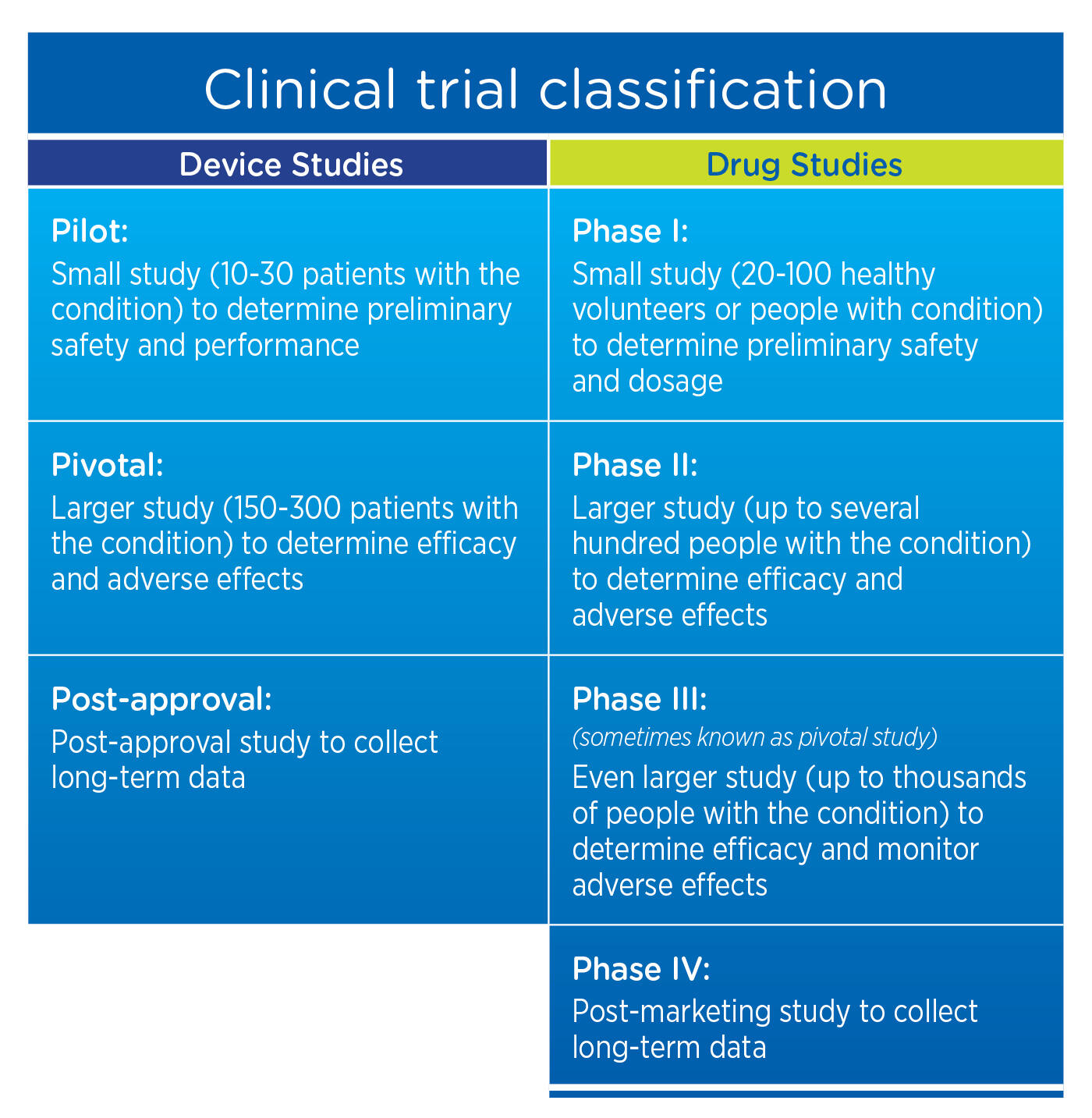
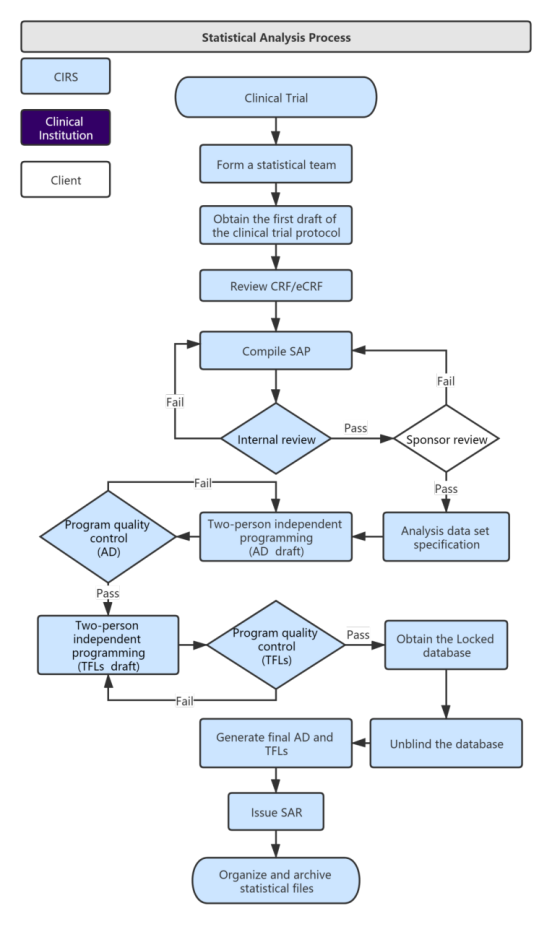
Clinical Evidence Requirements for CE certification under the Diagnostic Regulation in the European Union In-Vitro
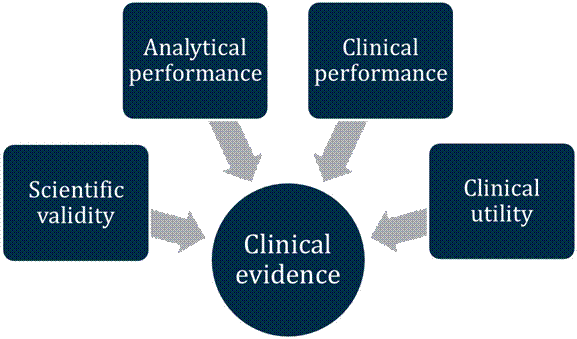
Clinical evidence guidelines supplement: In vitro diagnostic (IVD) medical devices | Therapeutic Goods Administration (TGA)

In Vitro Diagnostic (IVD) Development | Clinical Research Associate CRA - Career, Jobs, Certification, Industry Insight.

ISO 20916:2019(en), In vitro diagnostic medical devices — Clinical performance studies using specimens from human subjects — Good study practice