
Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - Clinical Therapeutics
Large Clinical Trial of Hydroxychloroquine for COVID-19 Sponsored by Novartis After Reaching Agreement With FDA
E17 General Principles for Planning and Design of Multiregional Clinical Trials Guidance for Industry
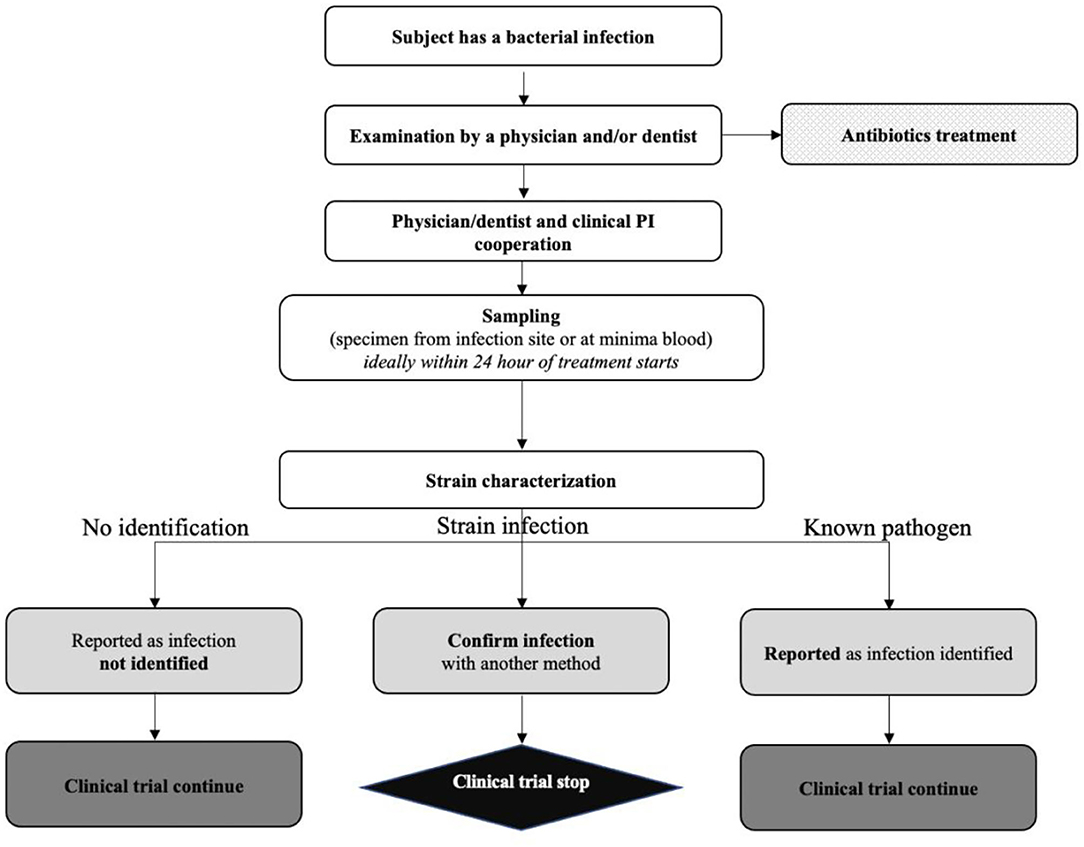
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA

Medical Device Development | Clinical Research Associate CRA - Career, Jobs, Certification, Industry Insight.



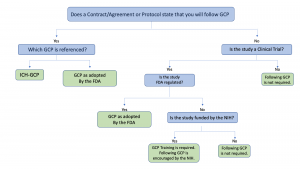





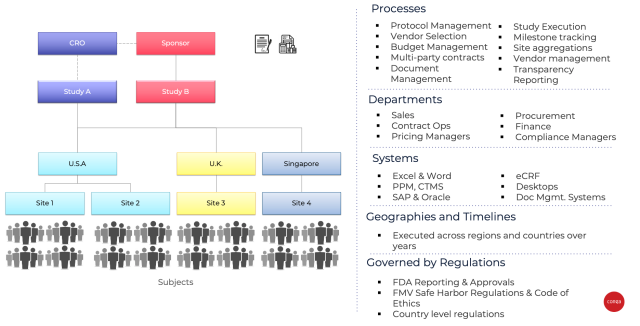
![FREE 10+ Clinical Trial Agreement Samples [ Site, Quality, Accelerated ] FREE 10+ Clinical Trial Agreement Samples [ Site, Quality, Accelerated ]](https://images.sampletemplates.com/wp-content/uploads/2021/09/Standard-Clinical-Trial-Agreement.jpg)
